This Trastuzumab (Herceptin) ELISA Pharmacokinetic kit is designed to measure trastuzumab with high specificity and enhanced sensitivity.
$809.99
Trastuzumab (trade name Herceptin®) is indicated for the treatment of HER2-positive breast cancer, and adjuvant therapies for metastatic gastric cancer and gastroesophageal cancer. Serum concentration of trastuzumab may predict some clinical outcome during therapy. It is also possible that the surveillance of circulating trastuzumab concentration during therapy represents a direct factor for immunogenicity and some other side effects. Identification of biomarkers and risk factors for adverse drug reactions that might be related to serum concentrations, and maintaining the effective minimum concentration of trastuzumab in order to potentially avoid some side effects, might be beneficial using a reliable method.
The Trastuzumab ELISA kit is designed to measure trastuzumab with high specificity and enhanced sensitivity. The assay design utilizes a pair of antibodies that allows the detection of the whole trastuzumab molecule in biological matrices.
This assay employs the sandwich enzyme immunoassay technique. Anti-Trastuzumab is coated onto a 96 well microplate. Calibrator, quality control samples and test samples are pipetted into the appropriate wells. Trastuzumab present in biological matrices is bound by the immobilized capture antibody. After washing away any unbound substances, enzyme linked detection antibody is added to the wells. The plate is washed to remove any unbound antibody-enzyme reagent and a substrate solution is added to the wells for color development. The color development is proportional to the amount of Trastuzumab present in test samples.
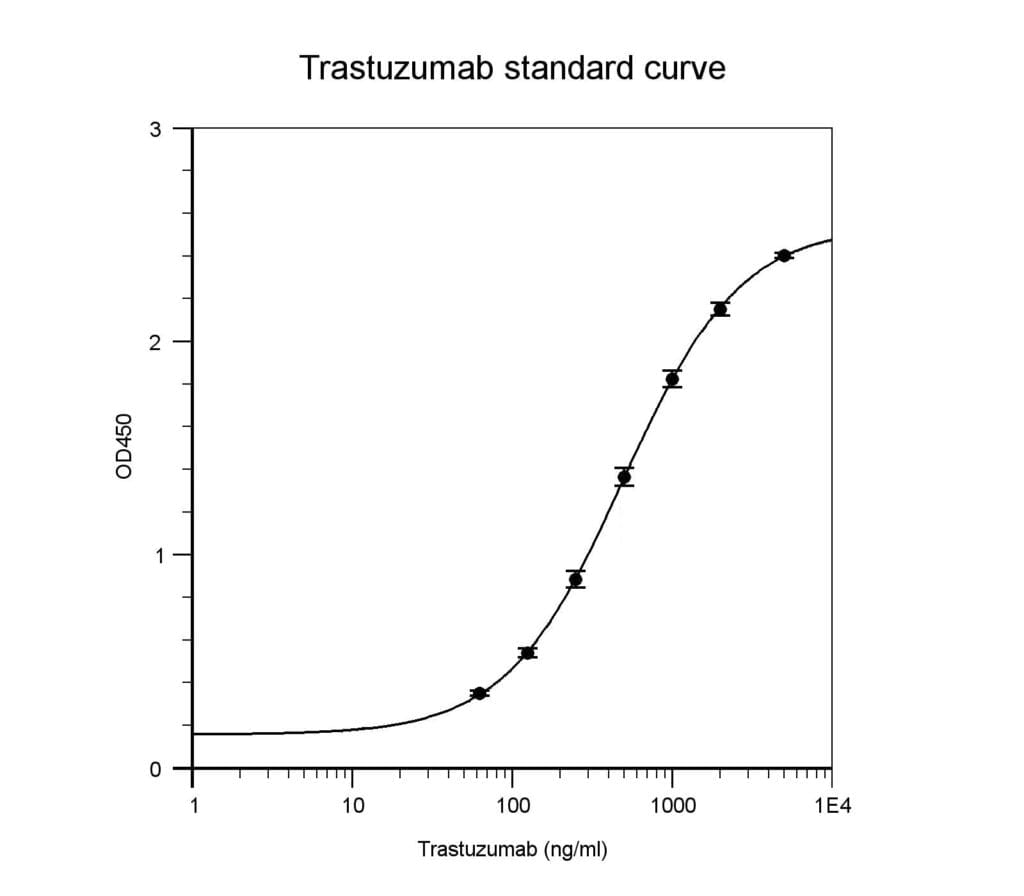
The standard curve was generated with the supplied calibrator using the recommended
conditions. Each sample was run with 6 replicates. Intra-assay coefficient of variance is <10%.

Subscribe to receive product updates & promotions.
Reviews
There are no reviews yet.