This Tocilizumab (Actemra®) ELISA kit allows researchers to measure anti-tocilizumab antibodies in biological matrices. This immunogenicity assay employs the bridging ELISA technique. Capture antibody (Tocilizumab) is pre-coated onto a 96 well microplate.
$809.99
Tocilizumab (Actemra® ) is a humanized recombinant monoclonal antibody used for the treatment of inflammatory types of arthritis, such as rheumatoid arthritis (RA). Tocilizumab works by binding and blocking the activity of the IL6 receptor. This kit allows researchers to measure anti-tocilizumab antibodies in biological matrices.
This immunogenicity assay employs the bridging ELISA technique. Capture antibody (Tocilizumab) is precoated onto a 96 well microplate. Quality control and test samples are pipetted into the appropriate wells. Anti-tocilizumab present in biological matrices is bound by the immobilized capture antibody. After washing away any unbound substances, secondary antibody is added to the wells and after a final wash a detection reagent is added. The plate is washed to remove any unbound antibody-enzyme reagent and a substrate solution is added to the wells for color development. The color development is proportional to the amount of anti-tocilizumab present in test samples. QC samples give a quantitative reference signal which can be
used to determine the level of anti-tocilizumab antibody in the unknown samples. The color development is stopped and the intensity of the color is measured.
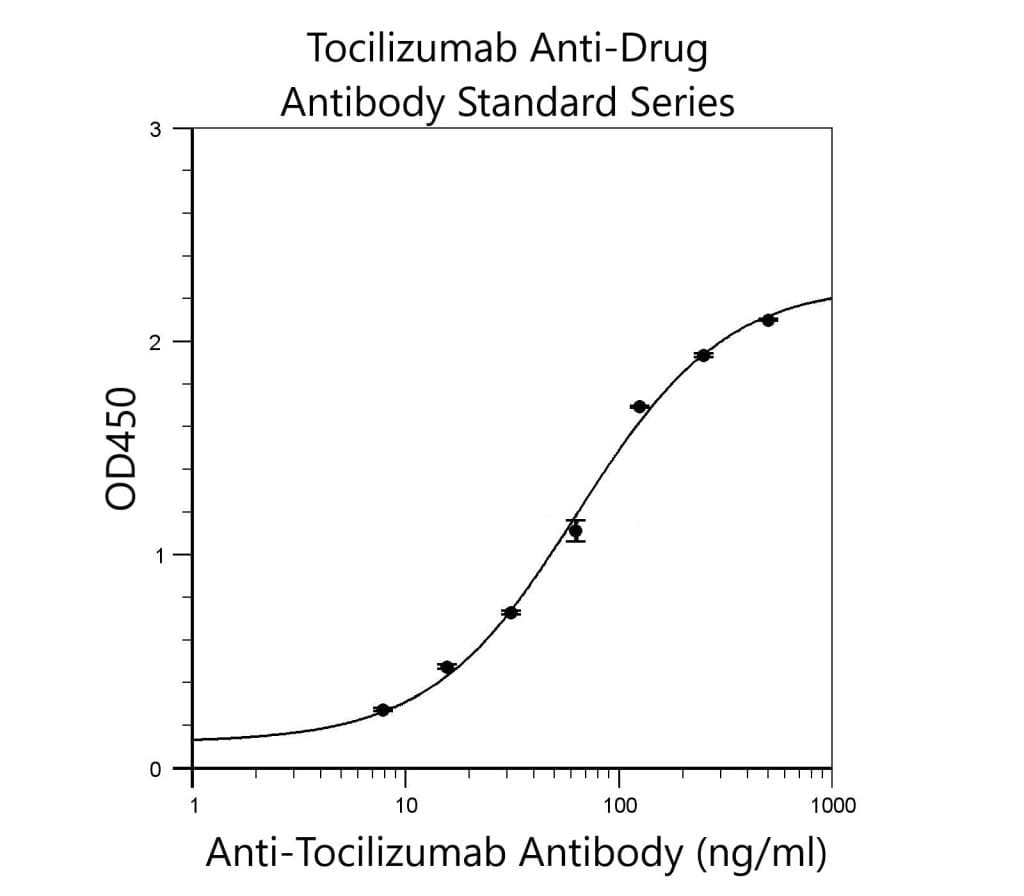
6 replicates of each QC sample diluted 1 in 10 and
then used to generate a standard series ranging
from 500ng/ml to 7.8ng/ml anti-Tocilizumab. Assay
developed for 6 minutes in this example.

Subscribe to receive product updates & promotions.
Reviews
There are no reviews yet.