Although several COVID19 vaccines have been approved and population immunity rates are increasing, it will still be a long time until everyone is immune. Until then, treatment with therapeutics remains an important clinical tool. Currently, there has been a slow uptake of monoclonal antibody therapies, including bamlanivimab and etesevimab (Lilly) and VIR-7831 (Glaxo Smith Kline), likely due to the expense and complexity of administering an infused drug in a hospital or treatment center during a pandemic.1
Another treatment option is repurposing existing small molecules already approved to treat other viruses. This approach avoids Phase I safety and tolerability in healthy volunteers and can move a candidate drug right to Phase II and III trials to show efficacy in COVID19 and confirm safety in a larger cohort of patients, respectively.2 The repurposed drugs being investigated belong to several classes: anti-viral agents that inhibit RNA polymerases, anti-inflammatory molecules, and protease inhibitors required for viral entry and replication.
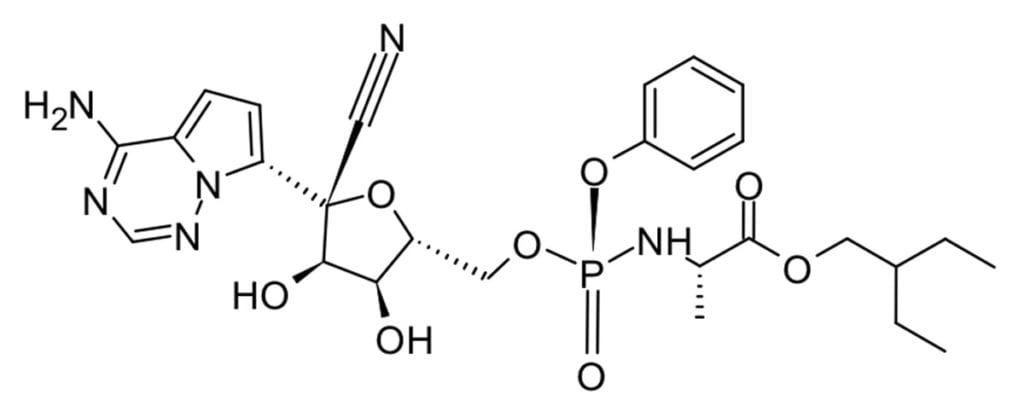
Remedesivir, an RNA polymerase inhibitor originally developed as a Hepatitis C treatment by Gilead, was an early star in the pandemic and it received emergency use authorization from the FDA in May 2020. It showed a slightly shorter recover time vs placebo (11 days vs 15 days) and decreased mortality (from 11.6% to 8.0%) just outside statistical significance.3
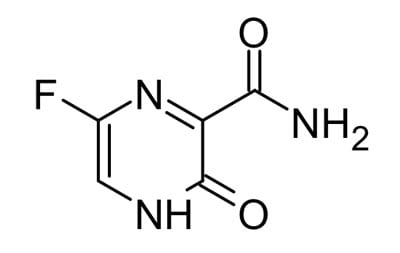
Favipiravir, another RNA polymerase inhibitor, has demonstrated broad anti-viral activity against influenza and ebola and it has the added advantage that it can be administered orally. However, preliminary results show only a small benefit from Favipiravir.2
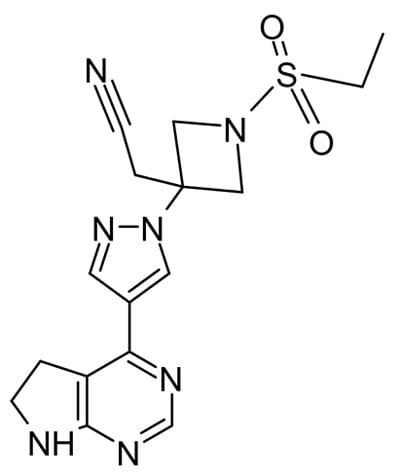
Other antiviral agents work by inhibiting enzymes other than polymerases critical for virus proliferation. In a recent randomized trial baricitinib, a protein kinase inhibitor, plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID19, particularly for those receiving high-flow oxygen.4
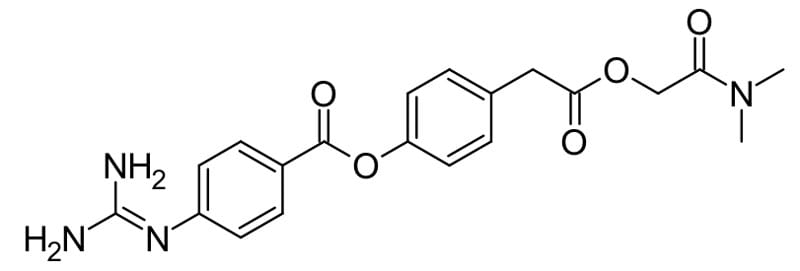
Camostat, a serine protease inhibitor blocks SARS-CoV-2 virus fusion with the cell, has been shown to reduce mortality in mice following infection from 100% to 30-35% at concentrations that would be clinically achievable in humans.5 A clinical trial is currently underway to evaluate camostat on COVID19 infection. A combination of protease inhibitors, lopinavir and ritonavir, originally used in HIV treatment has been found to be ineffective at treating COVID19.2
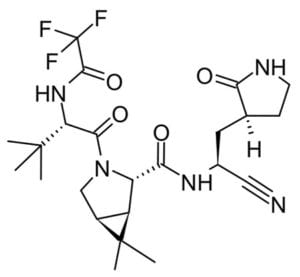
The majority of small molecule treatments have focused around re-purposing existing viral therapeutics. However, as it is becoming increasingly obvious that the COVID19 pandemic is far from over, perhaps it is time to focus on novel small molecules specific to SARS-CoV-2 such as Pfizer’s oral antiviral clinical candidate PF-07321332.⁶

Subscribe to receive product updates & promotions.